Redoxme
PTFE BASIC ELECTROCHEMICAL CELL SETUP
PTFE BASIC ELECTROCHEMICAL CELL SETUP
This cell is a sister version of BEC 50 mL. In this cell concept, all elements that stay in contact with an electrolyte or its fumes are made of PTFE and other fluorinated materials (tubing, fittings, and O-Rings). This cell is used wherever PEEK fails, i.e. when using electrolytes containing:
- Aqua regia - HNO3 + HCl
- Benzenesulfonic acid - aqueous C6H5SO3H
- Bromine (liquid) - pure Br2
- Carbolic Acid (Phenol) - C6H5OH
- Carbon Disulfide - CS2
- Chlorine (aqueous and anhydrous liquid) - Cl2
- Chlorosulfonic acid - pure ClSO3H
- Chromic Acid - aqueous H2CrO4 >50%
- Ethylene bromide (anhydrous) - pure CH2CHBr
- Fuming sulphuric acid (Oleum) - pure H2SO
- Hydrobromic acid - aqueous HBr
- Hydrofluoric acid - concentrated aqueous HF
- Nitric acid - aqueous HNO3 >50%
- Sulphuric acid - concentrated (96%) H2SO4
- Sulphurous acid - aqueous H2SO3
The chamber is also alkali-resistant, so it can be used instead of glass in experiments including high pH hot electrolytes. A full description of the difference between PEEK and PTFE cells can be found in our blog post.
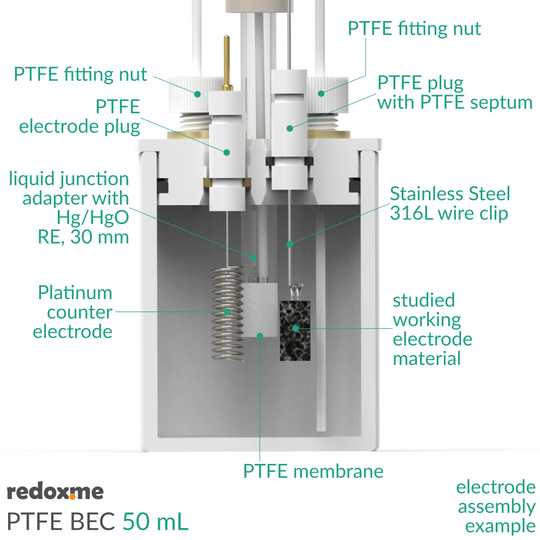
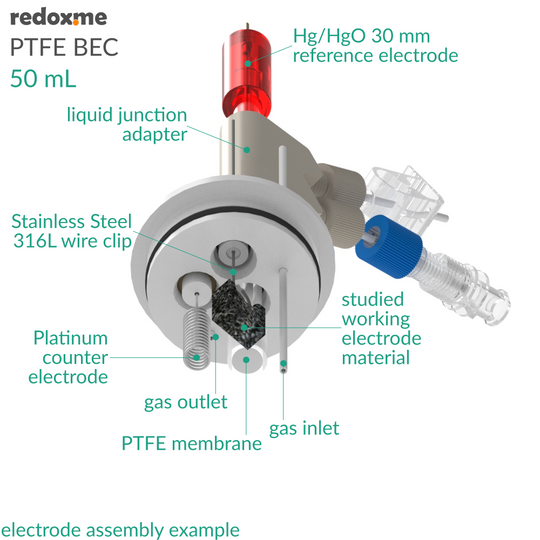
This is a stationary solution basic electrochemical (electrolytic) cell for measurements of electrodes in a form of: a) rod/disc (6 mm dia.), b) thin film deposited on flat substrate (using a wire clip) and c) membrane (using a wire clip). The working, counter and reference electrodes are mounted in a top casing either in 2-, or 3-electrode setup. When the reference electrode has a glass body, a liquid junction adapter is needed. The cell elements are constructed with materials that are inert to the sample (PTFE). It well fits aqueous (FKM O-Rings) and organic solvent (FFKM O-Rings) electrolyte requirements. The construction is gas-tight and can be used when the removal and exclusion of contaminants such as oxygen and water is required by bubbling of an inert gas through the electrolyte. The cell chamber is available in several material variants here.
Application note:
The reference electrode (or liquid junction adapter insertion tube) tip should be placed at the level of the working electrode center. This will ensure a low potential drop throughout the electrolyte solution for low-current experiments. Various auxiliary electrodes are suitable for this cell including metal wire and metal foil electrodes as well as graphite rod. The bubbling of gas through the solution must be stopped prior to experiment.
A typical cell setup configuration for strong acidic electrolytes includes:
- PTFE Basic Electrochemical Cell 50 mL
- PTFE 50HX15 0.6/250 wire counter electrode, Platinum
- 10 pcs of Stainless Steel 316L wire clip with PTFE septum plug
- Liquid Junction adapter with membrane mount
- Ag/AgCl 30 mm Silver / Silver Chrolide reference electrode
- PTFE membranes (PVDF in case of HF)
A typical cell setup configuration for strong (hot) alkaline electrolytes includes:
- PTFE Basic Electrochemical Cell 50 mL
- PTFE 50HX15 0.6/250 wire counter electrode, Platinum
- 10 pcs of Stainless Steel 316L wire clip with PTFE septum plug
- Liquid Junction adapter with membrane mount
- Hg/HgO 30 mm Mercury / Mercury Oxide reference electrode
- PTFE membranes
Specification
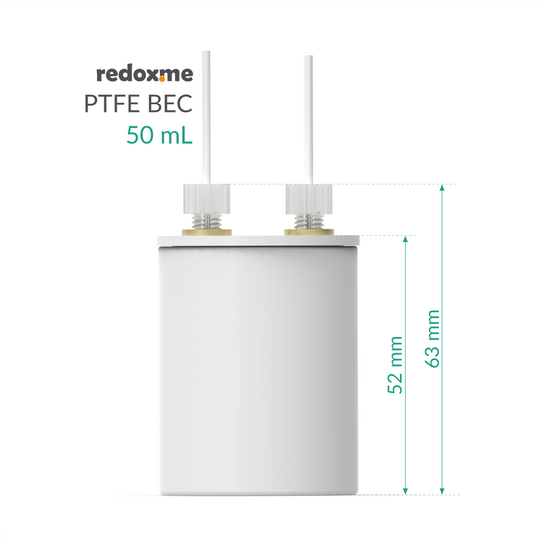
maximum electrolyte volume: 35 mL
electrode plug diameter: 6 mm
Intrastat data
HS Code: 90309000
Country of Origin: Sweden
NET weight: 200g
Setup includes:
1 x PTFE Basic electrochemical cell - PTFE BEC 50 mL
1 x PTFE lid
1 x PTFE chamber
1 x PTFE plug
1 x PTFE 50HX15 0.6/250 wire counter electrode, Platinum
1 x Reference electrode
1 x 10 pcs of Stainless Steel 316L wire clip with PTFE septum plug
Share